Why is deoxygenation (degassing of DO) necessary?
The presence of dissolved oxygen (DO) in water (or any liquid) causes a variety of adverse effects. In food products, for example, it promotes oxidation-induced deterioration, leads to loss of flavor, aroma and color, and facilitates bacterial growth — all of which ultimately forces manufacturers to set shorter expiry dates.
And in the case of chemical reactions, it can cause unintended reactions, inhibit desired reactions, corrode equipment or the inside of piping, present an explosion risk, or degrade the product under manufacture.
Methods for removing DO from liquid can be broadly categorized into chemical techniques and physical techniques.
The former involves using reducing agents such as sodium sulfite and hydrazine. Sodium sulfite is well-known as an antioxidant in wine.
The latter involves using a vacuum, heating, passing the liquid through a membrane, or introducing an inert gas such as nitrogen.
Of these, the most widely adopted is nitrogen stripping, which uses an inert gas (nitrogen) to displace the dissolved oxygen. This method creates no byproducts and therefore does not affect water quality, is safe and easy to handle, and moreover is relatively inexpensive.
Conventional methods bubble large volumes of gas over long periods
But simply bubbling is exceptionally inefficient
First, let us explain the basic principle behind nitrogen stripping.
Please take a look at the figure on the right.
High-purity N₂ gas, containing almost zero oxygen, is introduced into liquid containing DO (dissolved oxygen). (❶)
This is to create an oxygen concentration gradient (difference in partial pressure).
If you imagine the gradient as a slide and the oxygen as a person, it is the same idea as this: the steeper the angle of the slide, the more quickly they will slide down.
Oxygen moves from areas of higher concentration (the liquid) to areas of lower concentration (the bubbles of nitrogen). (❷)
Thus forms a combined nitrogen–oxygen gas, which is released from the surface of the liquid into the atmosphere, reducing the concentration of oxygen in the liquid. (❸❹)
However, simply bubbling N₂ gas will result in nothing but coarse bubbles. Such bubbles have only a small contact area with the liquid phase and rise rapidly, with an extremely short contact time. As a result, even when highly-concentrated N₂ gas is introduced, the vast majority of it will be wasted, escaping into the atmosphere with no accompanying oxygen.
For this reason, particularly when the target DO concentration is extremely low, it used to be necessary to bubble large volumes of N₂ gas for multiple hours. Some sites are even spending up to 24 hours.
Many sites have issues with exceptionally long lead times on the production line and an inability to increase production, all caused by such time-consuming and arduous deoxygenation processes.
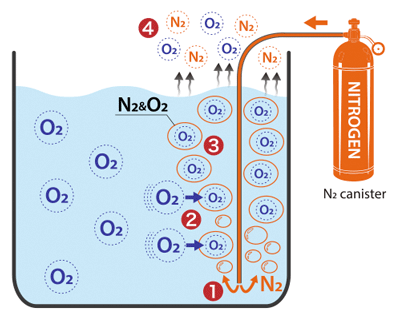
How nitrogen stripping works
- ❶ High-purity N₂ gas is introduced into the liquid from a canister or other source
- ❷ An oxygen concentration gradient forms. Oxygen moves from higher-concentration areas (the liquid phase) to lower-concentration areas (the nitrogen bubbles).
- ❸ Oxygen and nitrogen form a combined gas
- ❹ This combined gas is released from the surface of the liquid, lowering DO concentration
The OHR method: super-efficient deoxygenation treatment
The OHR method makes solving these issues all but effortless.
First, please have a look at this video, which compares the OHR method against the bubbling method in terms of deoxygenation efficiency in a tank containing 720 L of tap water.
The OHR method was able to reduce N₂ gas usage by 70.9% and treatment time by 56.3%.
Treatment time can be further shortened by using a larger model.
Next, please take a look at the graph below.
If, for example, you wish to deoxygenate from a DO of 10.0 mg/L down to 2.0 mg/L (that is, an oxygen removal rate of 80%), then a single pass through the OHR MIXER will suffice. It takes a mere 0.04 s for the fluids to pass through the OHR MIXER, during which short time it can achieve an oxygen removal rate of 80%. What is more, DO falls to 0.00 mg/L in approximately two passes.
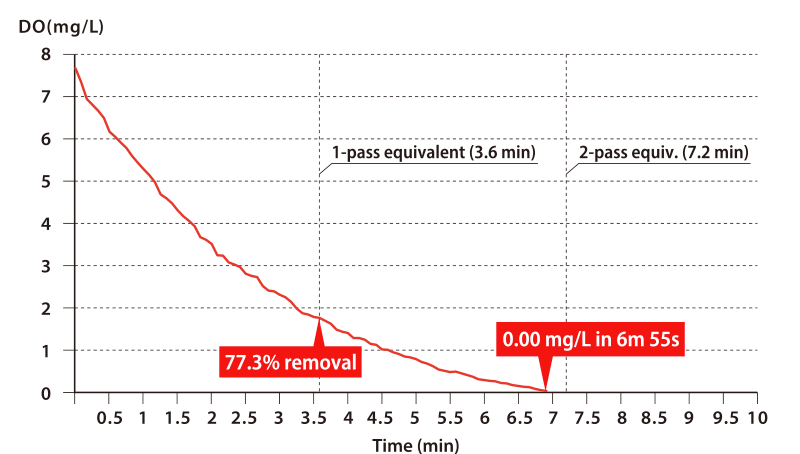
* This data is for batch processing carried out in a tank containing 720 L of tap water.
Water was sent to an OHR MIXER model MX-E25 using a pump capable of supplying 200 L/min.
A simple calculation gives a time of 3.6 minutes for the entire 720 L to complete one pass through the OHR MIXER, after which time the DO removal rate was 77.3%. Two passes then reduced the DO to 0.00 mg/L.
* In another test, in which pure single-pass processing was performed without the use of a tank (i.e. the tap water was fed directly into the pump suction line), an initial DO of 9.9 mg/L fell to 1.9 mg/L — a removal rate of 80.7%.
At one particular chemical plant, introducing the OHR method reduced N₂ gas usage by 79% and treatment time by 61%.
Annual expenditure on N₂ gas fell by some $17,500 USD, and production efficiency improved greatly.
The OHR MIXER requires absolutely no complicated operation, chemical additives or the like.
Simply assemble an OHR MIXER, a pump and N₂ gas, and the speedy deoxygenation can begin.
For more details, please contact us for our other explanatory materials.
10.0 mg/L → 0.2 mg/L DO is also possible via continuous processing
It is also possible to reduce a DO of 10.0 mg/L down to around 0.2 mg/L via continuous processing rather than batch.
In other words, it is possible to continuously deoxygenate incoming liquid and send it on to the next stage of processing, instead of circulating the liquid within a holding tank.
Please contact us for more information.
How is the OHR method able to achieve such highly-efficient deoxygenation?
Our one-of-a-kind reactor breaks down and repeatedly collides both the water and N₂ gas
The key is our one-of-a-kind reactor, the OHR MIXER.
It possesses such incredibly powerful mixing that its ability to refine particles is on par with that of a high-pressure emulsifier — while
operating at just 1% of the pressure.
The OHR MIXER harnesses this power to break down into fine particles not only the N₂ gas, but also the liquid containing the DO; it then
brings both into dense contact by violently colliding them with each other — all of this in the mere 0.04 s they take to pass through.
This drastically increases the efficiency with which the DO moves into the nitrogen bubbles, enabling deoxygenation to be completed in an instant.
The OHR MIXER has no moving parts and features a simple construction consisting solely of two fixed unique structures. Please have a look at this diagram.
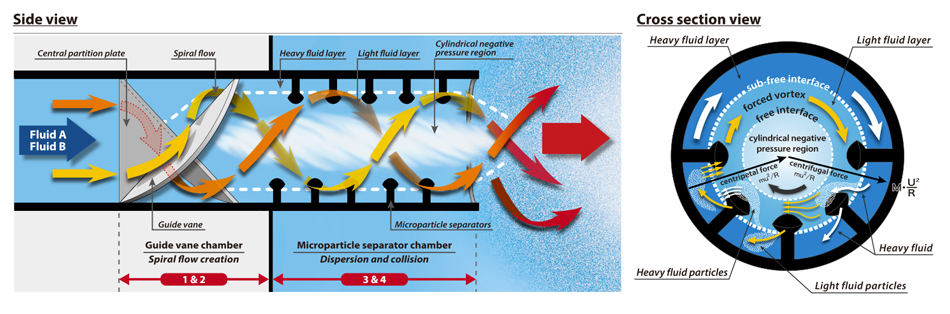
Both the nitrogen bubbles and the oxygen-containing liquid are broken down into fine particles, then collided with each other.
The dense contact this creates causes instant movement of oxygen.
This is why the OHR method has such high efficiency.












